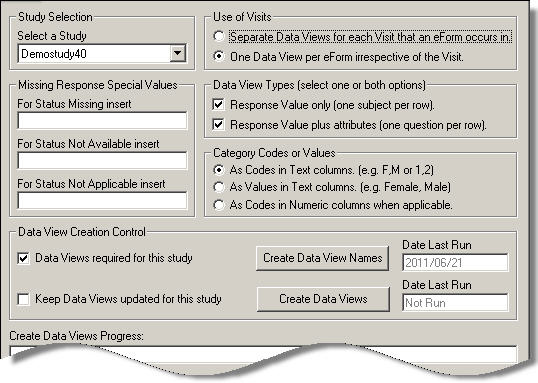
The first step in creating data views is to specify the structure of the extra tables which will be created in the database. This is done using the following options in the top half of the main Data Views screen. If any options are changed, the changes are only saved when the tables are next created.
Data view settings are specified individually for different studies. This allows you to set separate policies for each study. Select a study from the drop down list. If you only have one study in the database, it will be selected automatically.
This section enables you to specify how you want the visits to be organised. Separate database tables are created for each eForm, with the table name being automatically given a prefix which shows which type of data the table includes. Since an eForm can occur in more than one visit, you can choose whether to have a separate table for each visit that an eForm appears in, or just one table for all the eForm's data from all the visits. Select one of the two options:
Choose this option if you want to create a separate table of eForm data for each visit that contains a given eForm. Each data view table will be named in the following format:
StudyCode_VisitCode_FormCode
For example, the name of the data view containing data from the adverse event eForm in the screening visit of the demo study would be:
demostudy_screening_adverse
If the adverse event eForm occurs in another visit, say followup, there will also be another table called:
demostudy_followup_adverse
This is the default option. Choose this if you want just one table for each eForm, containing data from all visits. Each data view table will be named in the following format:
StudyCode_FormCode
For example, the name of the data view containing data from the adverse event eForm in the demo study (from all visits) would be:
demostudy_adverse
This section enables you to specify the structure of the data within each table. Select one or both of the two options by checking the required check boxes. If both are checked, both sets of tables will be produced.
This is the default option. Each row of the database will contain all the eForm's question responses for a particular subject. The table includes one column for each question, so each table will have a different number of columns depending on how many questions there are on the eForm. There are also columns to identify the subject, visit, eForm and question.
In this case, separate tables will also be created for each question group on an eForm, with each table including a column for each question in the question group.
Each eForm table will contain the following standard columns in the following format:
|
ClinicalTrialID |
Integer: internal MACRO study identifier |
|
Site |
The site code |
|
PersonID |
Integer: internal MACRO subject identifier |
|
VisitID |
Integer: internal MACRO visit identifier |
|
VisitCycleNumber |
The cycle number of a repeating visit (1 if not repeating) |
|
CRFPageId |
Integer: internal MACRO eForm identifier |
|
FormCycleNumber |
The cycle number of a repeating eForm (1 if not repeating) |
|
RepeatNumber |
The repeat number of a repeating question (1 if not repeating) |
In addition, there will be one column for each question, using the question code as the column header as follows:
DataItemCode1
DataItemCode2...and so on
The question response values will be stored in these columns.
Data view names created with this option will be prefixed with R, and are shown in the name listing as type RO.
If any eForms contain repeating question groups, additional tables will be created for each question group, so that a question group is treated just like a mini eForm.
With this option, the eForm table has a fixed number of columns, and each row represents a single response value for a particular subject. Each table will contain the standard columns described above, but with the following additional fixed columns displaying the attributes for each question. As a result, this option results in much longer tables than the other.
|
DataItemCode |
The question code |
|
ResponseValue |
The value of the response |
|
ResponseTimeStamp |
The time stamp of the response, showing the date and time of the entry |
|
DataType |
The question type, such as 'text', 'category', 'integer' and so on |
|
Units |
The unit of measurement that is linked to the question |
|
ValueCode |
The question code |
|
ResponseStatus |
The status of the question, such as 'Success', 'Warning', 'OK Warning', 'Missing' and so on |
|
LabResult |
The laboratory result, if relevant, either 'N', 'L' or 'H' |
|
CTCGrade |
The CTC grade, if relevant, either '1', '2', '3' or '4' |
Data view names created with this option will be prefixed with W, and are shown in the name listing as type WA.
For this option, the question repeat number is included as part of the row identifier, so extra tables are not created for repeating question groups on eForms.
Category questions require a response to be selected from a predefined list. You need to specify how you want these responses to be displayed. Each option in a category question has a unique code and a value.
If you select 'Codes in Numeric columns when applicable', this means that if a category question's codes are all numeric, a numeric column will be created (this cannot be done if any of the category codes are non-numeric).
The native database date/time data type does not support partial dates. This means that any date/time question that has been set by the study designer to allow partial dates will be represented in the data view table as a text column.
If a question has data missing, it will have been assigned one of three statuses; Missing, Unobtainable or NotApplicable. If you want to display these questions in your output, you need to specify a code for each status so that it can be indicated within the tables. Use numbers from -1 to -9 to avoid confusion with real data. The values you enter will be inserted where applicable in the tables.
Note: It is not possible for values -1 to -9 to be inserted in a date field so dates from 29/12/1899 (-1) to 21/12/1899 (-9) will be automatically inserted as missing values.
This section gives you a high level of control over how you want data views to work for the selected study.
Check this box to switch on the Create Data Views feature for the selected study. If the box is unchecked, you will be unable to create data view names or tables.
Check this box to keep the data view tables up to date by automatically adding new data to the relevant tables as it is entered into MACRO. This ensures that the data available for analysis is always current unless changes are made to the study definition itself, in which case the tables will need to be renamed and recreated.
If this box is unchecked, the tables will need to be updated manually as described in Step 3.
If you have already created tables with this box unchecked, the tables will not contain any data that has been entered since they were created. If you want to start updating the tables automatically, check the box and recreate the tables.
Once you have specified the table structure, you are ready to name the tables.