XML files containing archives of each subject are saved in the specified location as intermediate output. They are used to generate a set of HTML files for each subject, each contained within a separate folder. Each folder is named using the subject label. Any special characters used in subject labels will be replaced by underscores in file/folder names and HTML links. The data within the archived files themselves will still include the special characters in the subject label.
HTML output is stored in a folder named Studyname_timestamp in the location specified in the Archive tab of the Output settings.
The HTML output consists of the following information:
At the top of each page, the Study, Site, Subject identifier and Subject status are displayed.
Above each item is an item header identifying the item. The following may appear in the header, depending on the level of the item:
Visit name and cycle (if any)
Visit date
Visit status
eForm name and cycle (if any)
eForm date
eForm status
Data-item name*
Signature statement+
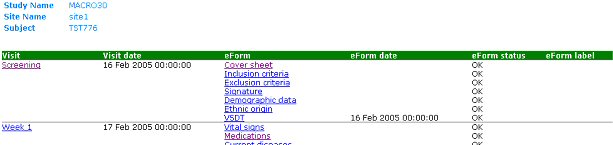
The schedule page, linked to from the subjects index page, shows all visits and eForms containing subject data. It appears as a table listing visit name and cycle (if any), visit date, eForm name and cycle (if any), eForm data, eForm status and eForm label. The visit name provides a link to visit SDVs. The eForm name provides a link to the eForm page. Beneath the schedule table is a table displaying subject level SDVs.
The eForm page, linked to from the schedule page, shows response values for the data-items on the eForm. It appears as a table per data-item listing the data-item name, signature statement (if an electronic signature question), response value, timestamp, database timestamp, user, software version, study version, status, reason for change (if applicable), reason for overrule (if applicable) and comment (if applicable). The data-item name provides a link to the response page. Beneath the eForm table is a table displaying eForm level SDVs.
The Response page, linked to from the eForm page, shows the response audit trail. It appears as a table listing the timestamp, database timestamp, response value, reason for change, overrule reason, user, software version, study version, status and comment. Beneath the audit trail table are tables displaying DCRs, notes and SDVs.
Multimedia attachments are copied from the Documents folder into the Attachments folder in the archive output folders. There will be an attachments folder for each HTML site/investigator folder. The output formats provide links to the attachments. Read-only files will not be copied but the user will be notified if there are any.