The following standard reports are provided
in the Reports folder within the MACRO application folder. Some of the
reports in this list are marked as sub reports and should only be accessed
via the relevant main report. Do not add links directly to sub reports
on your home page as the data displayed may not be accurate.
You can specify an image to be used at the
top of each standard report by adding the CompanyLogo
setting to the web.config file.
Report |
Target Name |
Target Location |
Description |
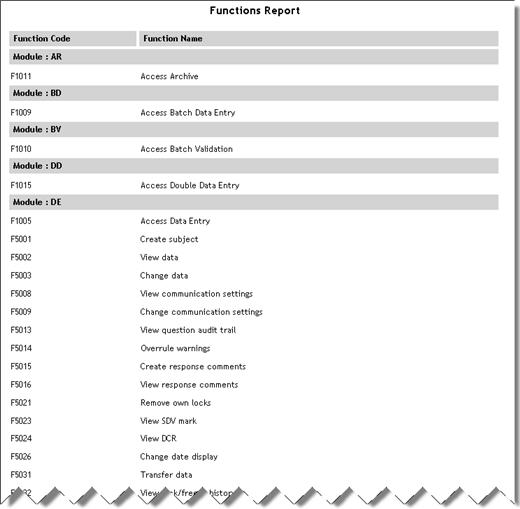
Security Functions |
Functions.aspx |
|
Lists
all functions carried out in each module in the currently open
database.
See example
|
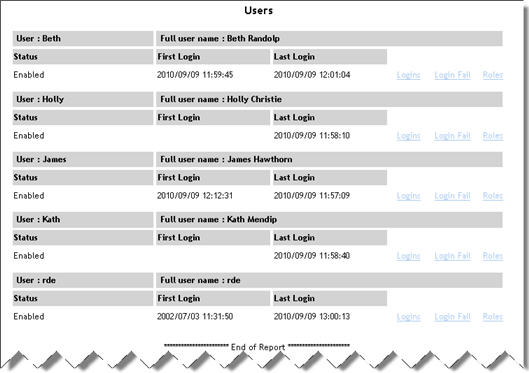
Users |
Users.aspx |
|
Lists
all users in the currently open security database. For each user,
the full username, status (enabled or disabled), first login and
last login are displayed.
Contains links to User Login Activity, Failed Login Attempts
and User Roles reports.
See example
|
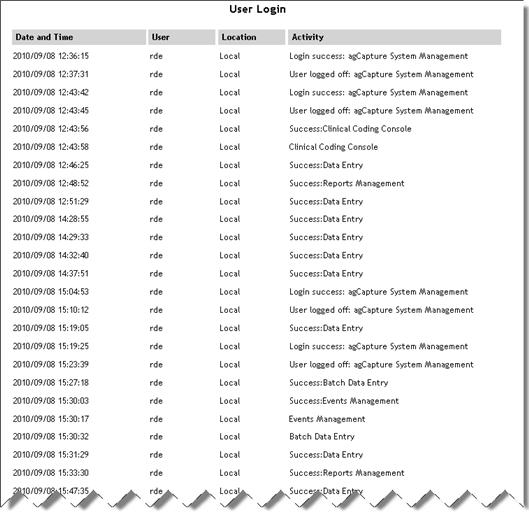
User Login Activity |
UserLogin.aspx |
|
Lists
all successful logins from earliest to most recent. For each login,
the date/time, user, location and activity are displayed.
See example
|
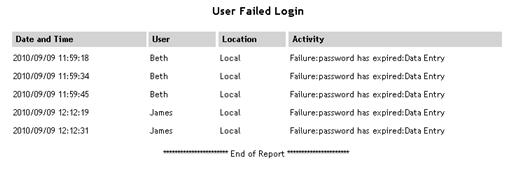
Failed Login Attempts |
UserLogin.aspx?failed=1 |
|
Lists
all failed logins from earliest to most recent. For each login,
the date/time, user, location and activity (reason for failure)
are displayed.
See example
|
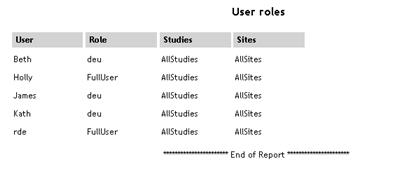
User Roles |
UserRole.aspx |
|
Lists
all users along with their user role and the studies and sites
with which they are associated.
See example
|
Report |
Target Name |
Target Location |
Description |
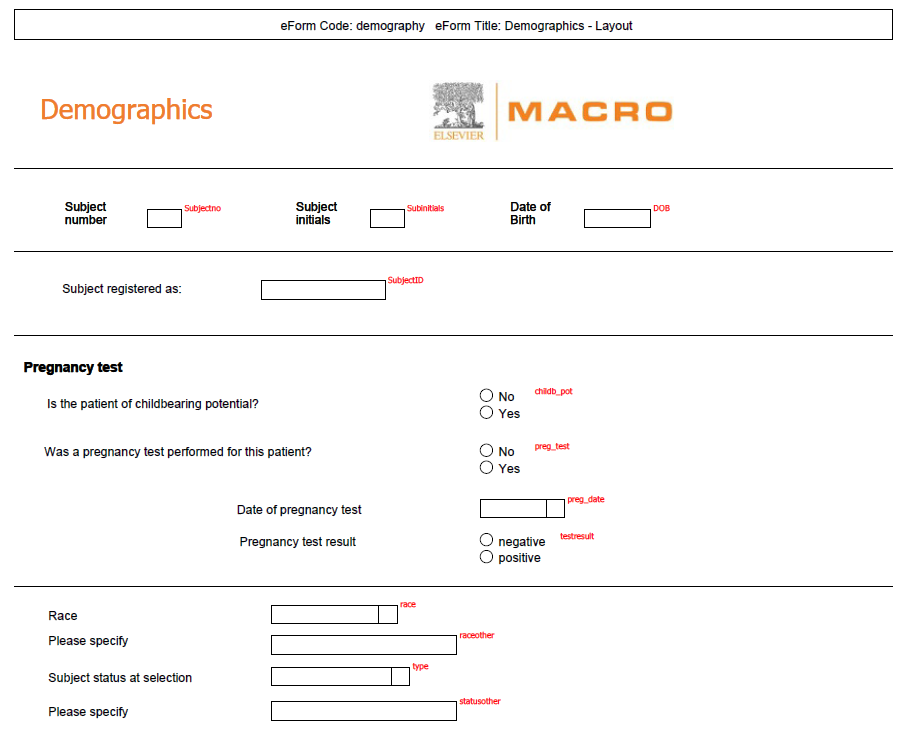
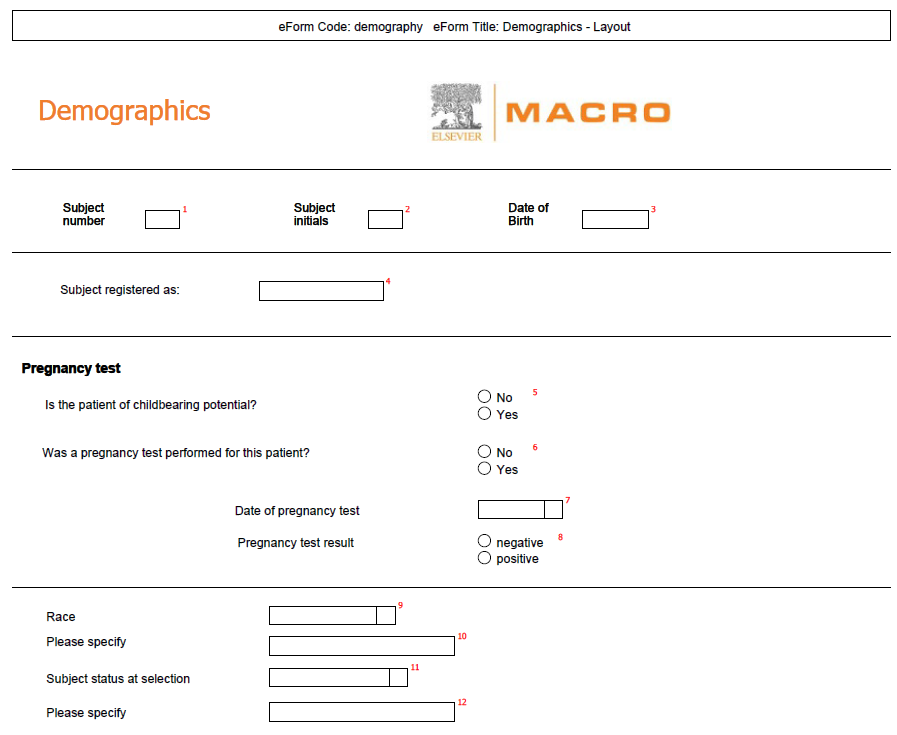
Annotated
CRF |
AnnotatedCRFReport.aspx |
|
Produces
a multi-page PDF report detailing the study definition with a
visual representation of the schedule and each eForm. Questions
are displayed with blank fields and are either labelled with the
question code to create a record for the study designer, or numbered
sequentially to create a paper form that can be used for manual
data entry. More
help...
See example using question codes
See
example using numbers
|
Study
List |
StudyList.aspx |
|
Lists
all studies in alphabetical order. For each study, the study description
and status are displayed.
Contains links to Study Details, Study Schedule, Study Questions,
Study Question Details, Study Validations and eForm Summary reports.
See example
|
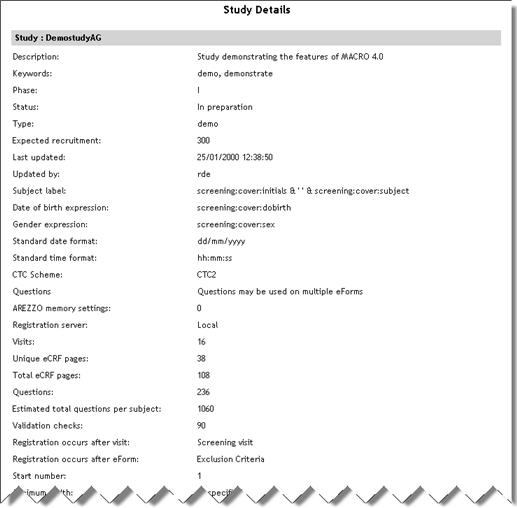
Study Details
(Sub report accessed
from Study List report) |
StudyDetails.aspx |
|
Lists
details of study taken from the study definition.
See example
|
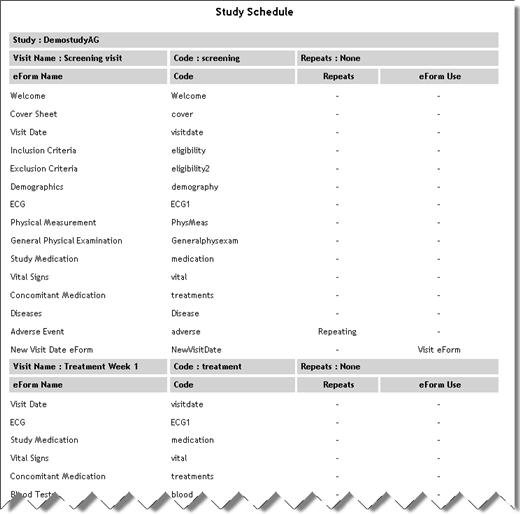
Study Schedule
(Sub report accessed
from Study List report) |
StudySchedule.aspx |
|
Lists
each eForm in the study with eForm code, repeat status and whether
the eForm is used in a special way such as a visit eForm.
See example
|
eForm Summary
(Sub report accessed
from Study List report) |
EformSummary.aspx |
|
Displays
a count of eForms being used in the study across different visits
planned as per visit plan. Data is grouped by study. |
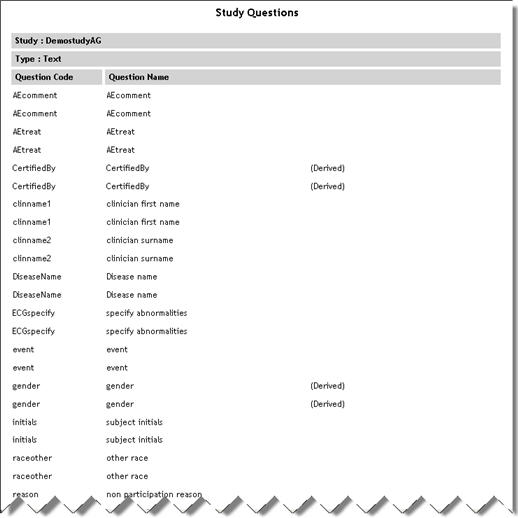
Study Questions
(Sub report accessed
from Study List report) |
StudyQuestions.aspx |
|
Lists
each question in the study with question name and any special
attributes such as whether it is derived.
See example
|
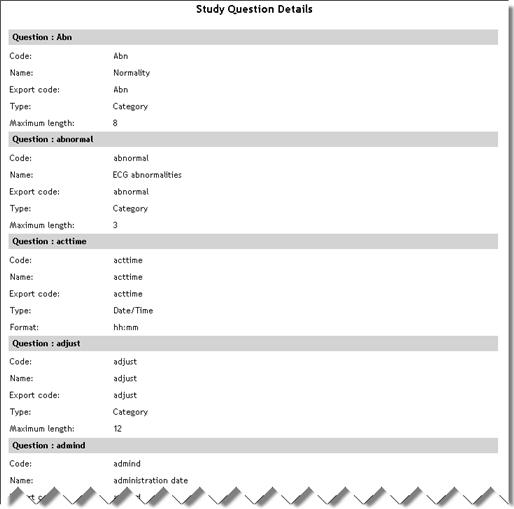
Study Question Details
(Sub report accessed
from Study List report) |
StudyQuestionDetails.aspx |
|
Lists
each question in the study with code, name, export code, type
and maximum length.
See example
|
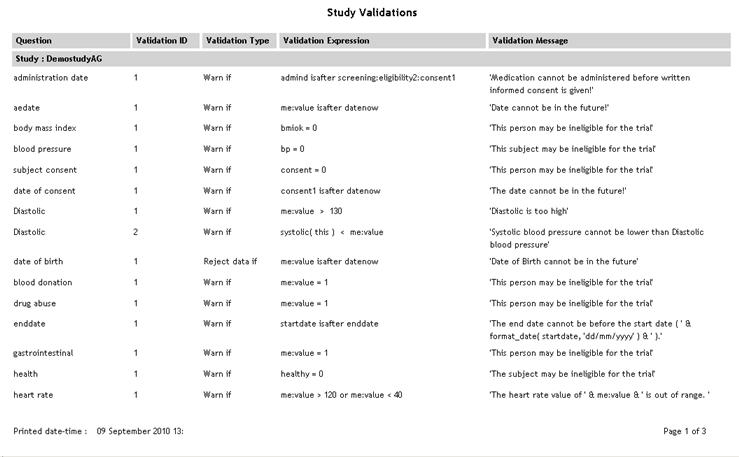
Study Validations
(Sub report accessed
from Study List report) |
StudyValidation.aspx |
|
Lists
each question in the study that has a validation attached along
with validation ID, type, expression and message.
See example
|
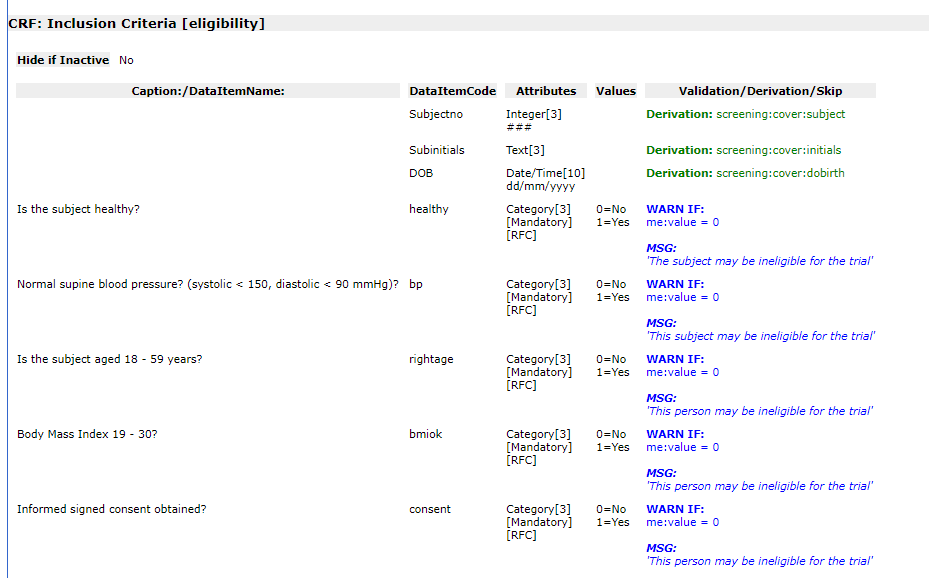
Detailed
Study Definition |
Detailed_study_definition.asp |
M3/Reports |
Lists
visits, eForms and questions in the order in which they appear
in the study. Includes study details and a visual representation
of the visit schedule. Visits and eForms are listed with labels,
dates and internal triggers. Each question is listed with name,
code, type, format, length, mandatory/optional, RFC, category
codes & descriptions, validation expressions & messages,
derivation expressions and collect-if conditions.
See example
|
Report |
Target Name |
Target Location |
Description |
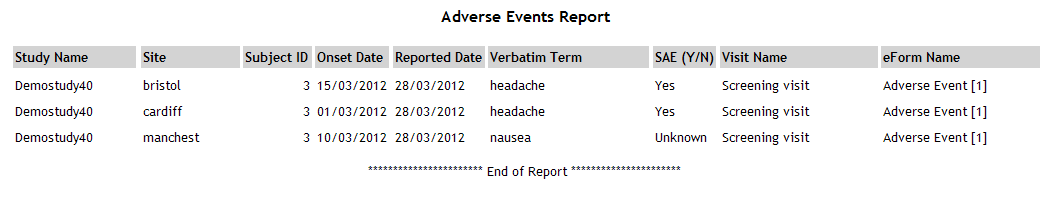
Adverse Events |
AdverseEvents.aspx |
|
Displays details of adverse events.
This report requires the following questions to exist in the study
definition:
- A date/time question to capture the date of onset with
the code AESTDAT and the format dd/mm/yyyy
A text question to capture
the adverse event description with the code AETERM and maximum
length 50 A category question to capture
the severity of the event with the code AESER and the values
N (No), NA (Not Applicable), U (Unknown), Y (Yes)
See example
|
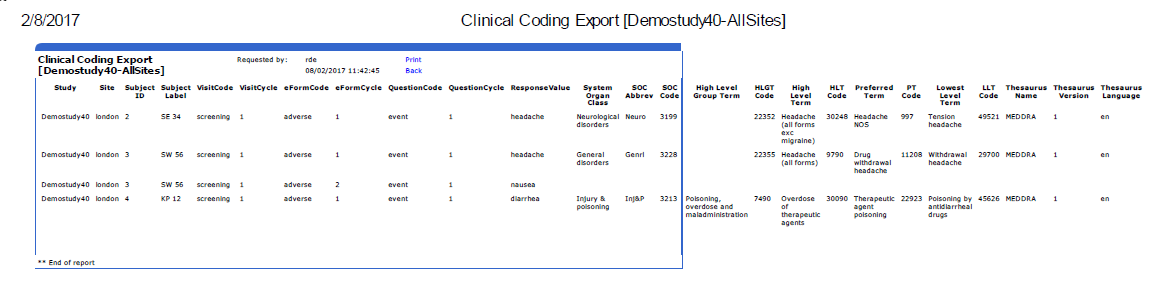
Clinical Coding Export |
ClinicalCoding_Export.asp |
M3/Reports |
Displays
the complete set of clinical coding information for all coded
questions at one or all sites for a single study. For each question,
it gives:
Response
value System
Organ Class, abbreviation and code High
Level Group Term, code and level term Preferred
Term and code Lowest
Level Term and code
See example
|
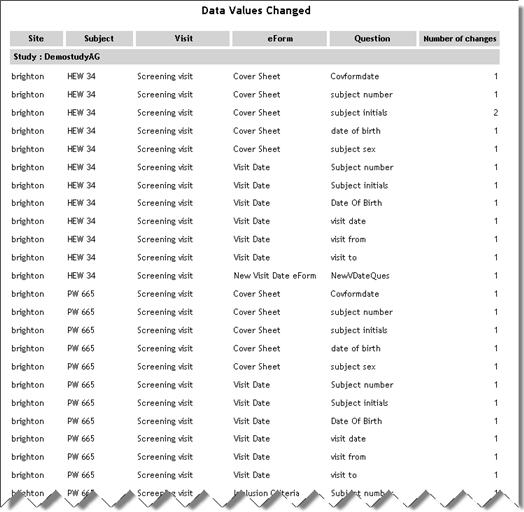
Data Values Changed |
ChangedDataValues.aspx |
|
Displays
responses to questions in the specified study that have been changed
more than the specified number of times and have multiple entries
in the audit trail.
See example
|
Missing Data - Study Summary |
MissingData.aspx |
|
For
each study, the number of missing values, total number of values
and % missing is displayed.
See example
|
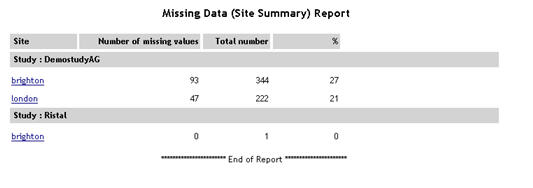
Missing Data - Site Summary |
MissingDataSite.aspx |
|
For
each study, a list of sites is displayed. For each site, the number
of missing values, total number of values and % missing is displayed.
See example
|
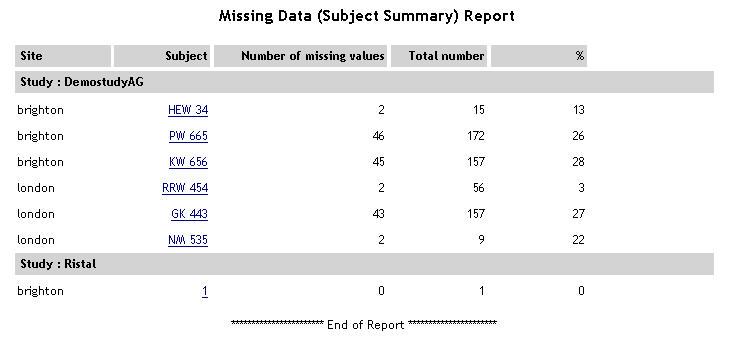
| Missing Data - Subject Summary |
MissingDataSubject.aspx |
|
For
each study, a list of subjects is displayed. For each subject,
the site, number of missing values, total number of values and
% missing is displayed.
See example
|
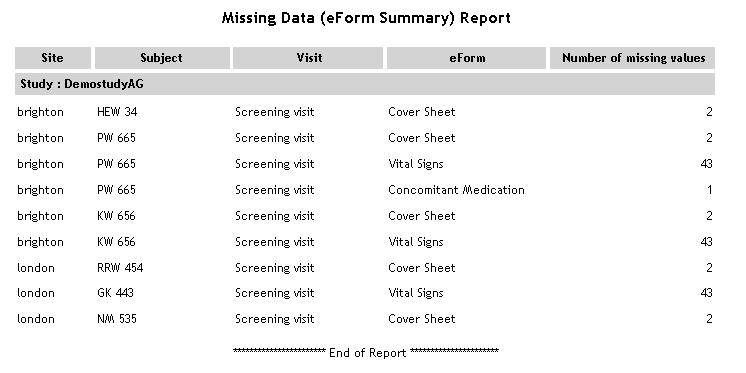
| Missing Data - eForm Summary |
MissingDataEform.aspx |
|
For
each study/site/subject, a list of eForms is displayed. For each
eForm, the number of missing values is displayed.
See example
|
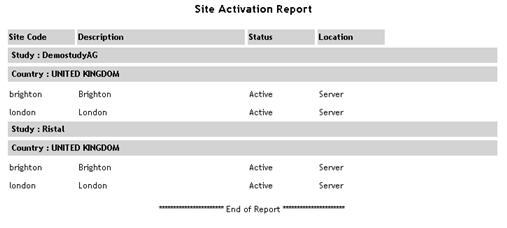
| Site Activation |
SiteActivation.aspx |
|
Displays
the current status (active or inactive) of all sites within the
specified study/country.
See example
|
| Site Recruitment |
SiteRecruitment.aspx |
|
Displays
the recruitment numbers for the specified study/country/site.
See example
|
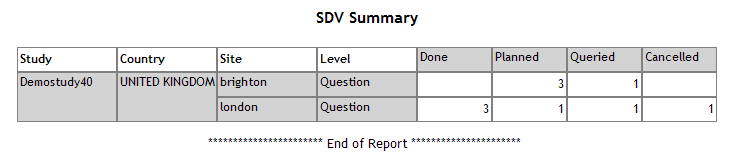
| SDV Summary |
SDVSummary.aspx |
|
Lists
all the data items (subject, visit, eForm and questions) and their
SDV status
and status date for the specified study/country/site.
See example
|
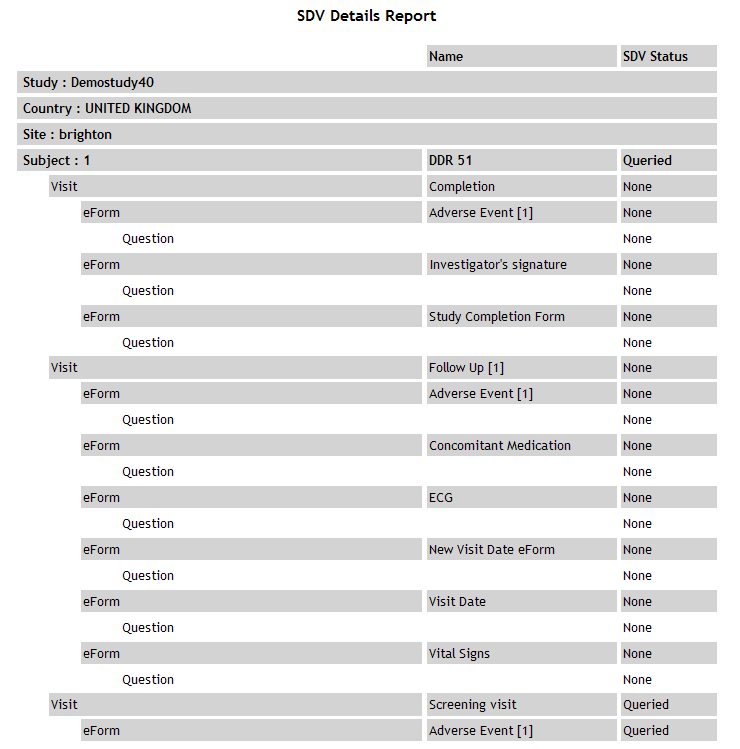
| SDV Details |
SDVDetails.aspx |
|
Lists
all questions with SDVs attached, displaying the number of SDVs
of each status for each question.
See example
|
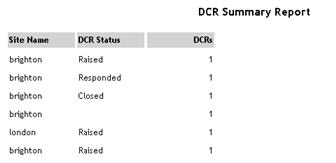
| DCR Summary |
DcrSummary.aspx |
|
Lists
the number of DCRs of each status for the specified study/country/site.
See example
|
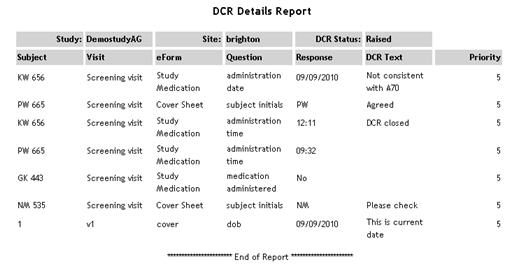
| DCR Details |
DcrDetails.aspx |
|
Lists
all DCRs in each study, showing subject/visit/eForm/question,
response value, DCR text and priority.
See example
|
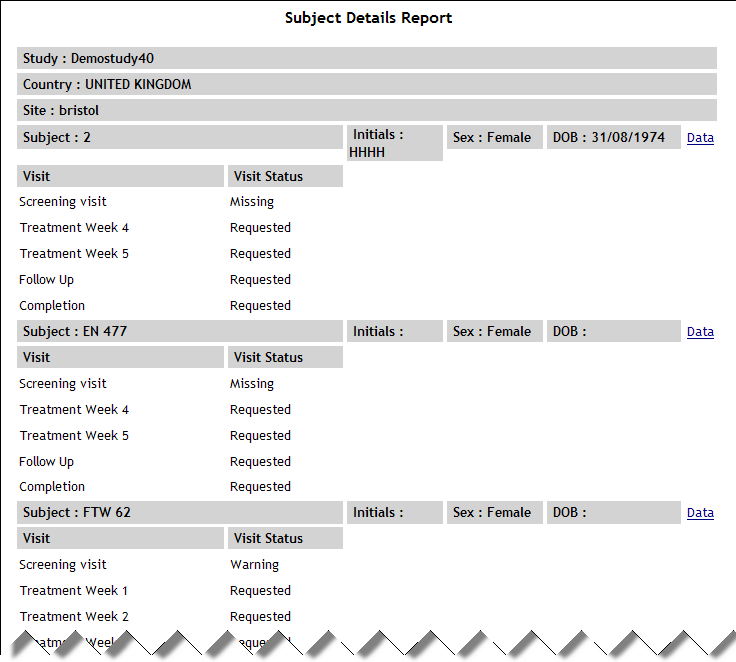
| Subject Details |
SubjectDetails.aspx |
|
Displays ID, initials, sex and
DOB for each subject in the specified study/country/site along
with the status for each visit in the schedule. This
report requires the following questions to exist in the study
definition:
A text question to
capture subject initials with the code SUBJINIT and maximum
length 4 A category question
to capture gender with the code SEX and the values F (Female),
M (Male), U (Unknown) A date/time question
to capture date of birth with the code BRTHDAT and the format
(dd/mm/yyyy)
See example
|
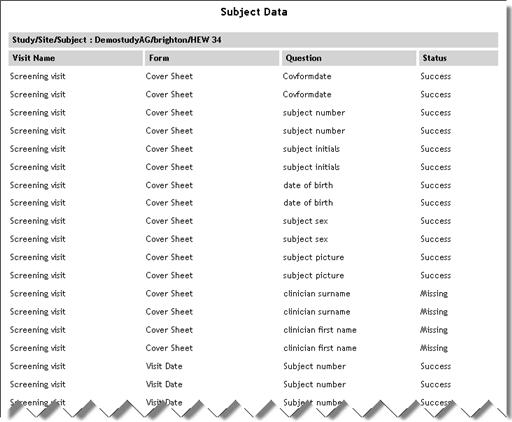
Subject Data
(Sub report accessed
from Subject Details report) |
SubjectData.aspx |
|
Displays
the status of each visit/eForm/question in the specified study/country/site.
See example
|
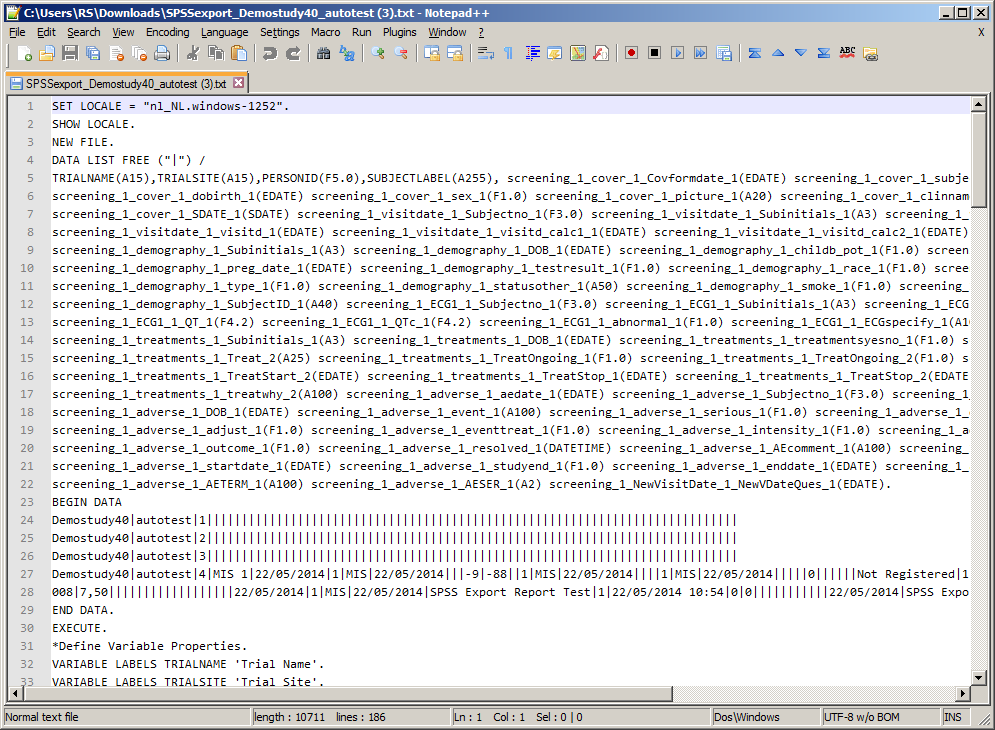
| SPSS Export |
SPSSexport.asp |
M3/Reports |
Displays
all unique Visit/eForm/Question combinations of subject data including
multiple repeats for a single site and enables you to download
the data in a format suitable for import into SPSS.
See
example
How
to run the report... |